In boiler systems, nearly all problems related to premature water- and fire-tube failure, unexpected performance loss, and system failure can be traced to water chemistry and treatment. Taking the steps to develop and maintain an efficient and reliable water-treatment program will ensure a successful boiler system.
With a boiler system, the importance of water treatment is determined largely by the type and application of the boiler. There are many types of boiler applications, the primary ones being hot water and steam.
Hot-Water Applications
In hot-water applications, boilers typically operate within a stable closed-loop system in which the water chemistry is fairly constant. This means water is treated and stabilized for use during the initial fill of the system. Periodic checks and minor chemistry modifications are needed to keep the loop under control, provided there are no major leaks introducing large amounts of raw makeup water.
In hot-water systems, water quality tends to be less of an issue and more predictable, which leads to tighter tolerances. In the light- and small-commercial market, the most common types of boilers include copper fin, cast iron, high mass, and, whether condensing or non-condensing, water tube. Fire-tube boilers are available in this market, but usually require more installation space than comparable-output water-tube boilers. In water-tube boilers, flue gases surrounding tubes heat water inside of the tubes. In fire-tube boilers, flue gases are inside of tubes, and water is in the shell around the tubes.
Steam Applications
In steam applications, water quality is an important part of boiler selection. Steam boilers tend to be steel water-tube- or fire-tube-type boilers in the 10-to-1,000-hp range. Fire-tube boilers are more robust and more tolerant of poor water quality, but require more physical space, than water-tube boilers.
Regardless of boiler type, water quality is a critical aspect of implementing and operating an efficient steam plant. Key components of steam-boiler water treatment include makeup-water treatment, oxygen removal, and feedwater treatment.
Mathematical Relationships
In most plants, steam losses can be significant, varying from 30 percent to 80 percent of total boiler output. These losses can be attributed primarily to leaks and processes that leave condensate in a contaminated or unrecoverable state.
Fresh makeup water is required to account for condensate losses in a system. Scale-forming minerals are removed as the first line of defense in controlling water chemistry and steam quality. This can be done through precipitation softening, ion exchange, or reverse osmosis. Makeup-water-supply quality, economics, and the end use of the steam often dictate which approach is most appropriate. For most commercial and light-industrial applications, an ion-exchange softening process is utilized to minimize costs.
Condensate return water is combined with treated makeup water and deaerator steam to produce boiler feedwater. This feedwater is introduced to the boiler, and mostly pure steam is boiled off. During the boiling process, all of the solids and impurities left behind in the boiler become concentrated. This requires blowdown or a deliberate bleeding off of the boiler water. With the blowdown having to be made up as well, the total makeup can be expressed as follows:
TM = SL + CL + BL
where:
TM = total makeup
SL = steam losses
CL = condensate losses
BL = boiler water losses
Makeup and blowdown can be represented in terms of a percentage of feedwater, when the flow rates of these streams are known. Their relationships are expressed as follows:
%BD = 100% × (BD ÷ FW)
%MU = 100% × (MU ÷ FW)
where:
BD = blowdown mass flow, pounds per hour (kilograms per hour)
MU = makeup mass flow, pounds per hour (kilograms per hour)
FW = feedwater mass flow, pounds per hour (kilograms per hour)
Often, mass-flow rates are not known, in which case the percentages can be calculated based on a ratio concentration of a dissolved solid or total dissolved solids (TDS) in the different streams:
%BD = 100% × (TDSFW ÷ TDSBD)
where:
TDSFW = TDS in feedwater, milligrams per liter
TDSBD = TDS in blowdown, milligrams per liter
The percentage makeup can be calculated as follows:
%MU = 100% × (TDSFW − TDSC) ÷ (TDSMU − TDSC)
where:
TDSC = TDS in recovered steam condensate, milligrams per liter
TDSMU = TDS in makeup, milligrams per liter
The quantity of dissolved solids in boiler water compared with that in feedwater can be described in a concentration ratio or cycles of concentration (COC). In the simplest terms, it is the reciprocal of the percentage blowdown:
COC = (100 ÷ %BD)
Often, when makeup water is demineralized, COC cannot be calculated using a conductivity probe. Fluorescing tracer chemicals can be utilized to determine the ratio of concentration of boiler water to feedwater. Another approach is to measure the steam being produced and the blowdown flow rate to calculate COC:
COC = (S + BD) ÷ BD
where:
S = steam mass flow, or boiler output, pounds per hour (kilograms per hour)
Removing Oxygen and Gases
Another important function of boiler-feedwater treatment concerns the removal of oxygen and dissolved gases, such as carbon dioxide and ammonia. The presence of these gases can result in corrosion that ultimately causes tubes to fail and piping to rupture and leak. Carbon dioxide primarily affects condensate systems, as it turns to carbonic acid, which can aggressively deteriorate components.
The primary mechanism for removing oxygen and dissolved gases is the deaerator. There are several methods of deaeration; selection depends on the size of the system, the type of gases, the concentration to remove, and economics. The most common types are spray, tray, and atomizing. For most small to medium-sized commercial installations, spray type is the most compact and economical.
Effective deaerators reduce oxygen levels in feedwater to about 0.007 to 0.04 mg per liter (7 to 40 μg per liter). Often, additional oxygen removal is accomplished with a chemical scavenger. Commercial oxygen scavengers on the market include:
- Sodium sulfite (Na2SO3).
- Hydrazine (N2H4).
- Carbohydrazide [(NH2NH)2CO].
- Erythorbate (RC6H6O6).
- Diethylhydroxylamine [(C2H5)2NOH].
- Methylethylketoxime (C4H8NOH).
- Hytroquinone [C6H4(OH)2].
In most commercial and light-industrial applications, sodium sulfite is used to control oxygen beyond the capabilities of deaerators. However, sulfite adds solids and contributes to the need for increased blowdown flows. Other scavengers can double as metal passivators, protecting equipment and piping, in addition to reducing oxygen levels. Many other compounds, however, break down into carcinogenic and acidic components.
Scale Control
Mineral scale is comprised largely of precipitates of calcium and magnesium salts. Although ion-exchange water softening largely removes scale-forming agents, feedwater should be monitored regularly to ensure none is being introduced from other sources.
Silica scale also can be a problem, although it rarely is found in systems below 600 psig. Silica deposits can form when boilers operate at excessive COC or pretreatment softeners are not designed to remove silica. For most applications in the western United States, where most water comes from the melting of mountain snow pack, mineral scale is the larger culprit.
Whereas scale associated with water hardness used to be the main cause of boiler failures, the push to return as much condensate as possible has led to increased iron deposits in boilers. Iron deposits on heat-transfer surfaces now are the predominant mode of failure. As deposits accumulate, they act as insulators, impairing heat transfer. This ultimately causes metal to overheat and fail. The porous nature of the deposits exacerbates the situation by trapping corrosive chemistries, such as caustic acid phosphates, sulfates, and chlorides. Unfortunately, even after significant research initiatives, the deposition mechanism of iron deposits on boiler surfaces is not well-understood.
Most iron originates from raw-source makeup water or the corrosion of mild-steel components within a steam and condensate system. Corrosion of mild steel is influenced by factors such as pH, temperature, heat flux, dissolved oxygen, carbon dioxide, flow, ionic strength, suspended solids, and boiler-treatment chemicals. Fortunately, mild steel at high temperatures under alkaline-reducing conditions, or high pH, will form a protective magnetite-oxide (FE3O4) layer on the surface, protecting the base material.
Special care should be exercised in monitoring and protecting oxides. As boilers and systems are taken offline and restarted, the expansion and contraction of metals can cause oxides to crack and flake off, exposing the base metal to continued corrosion. Additionally, caustics or acids can interact with oxides and cause them to break down. For this reason, the recommended pH is in the alkaline rage of 9 to 12.
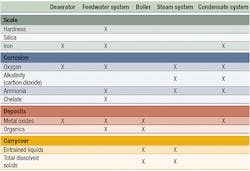
Table 1 summarizes common problems and affected areas.
Key Takeaways
Following is advice for developing a water-treatment program:
- Be willing to invest in a high-quality program from a reputable service provider. Good treatment programs can save costs attributed to shutdowns for tube or equipment replacement.
- Take a proactive approach. Actively engage a reputable water-treatment service provider to assist in the development of a program tailored to your system. Also, test the makeup-water source and any condensate or feedwater system, and include necessary components in the design.
- Consider that operators may not know how to maintain effective water-treatment programs. Have the water-treatment service provider conduct detailed training. Consider recording the training for future reference.
- Employ thorough monitoring procedures, frequently checking water chemistry at different control points within the system, and document results. Elevated temperatures can cause chemistries to go out of spec quite rapidly. Knowing the normal tolerance ranges and monitoring frequently (two or three times a day) can prevent scale and iron deposition from escalating beyond recoverable limits.
- Keep it simple. The simpler the design and program, the greater the likelihood operators will stick to it.
Reference
Flynn, D. (2009). The Nalco water handbook (3rd ed.). McGraw-Hill.
Corey Lehman, PE, is an associate principal and HVAC- and plumbing-system designer with Southland Industries, a national mechanical, electrical, and plumbing building-systems firm. He holds bachelor’s and master’s degrees in architectural engineering from The Pennsylvania State University. He can be reached at [email protected].
Did you find this article useful? Send comments and suggestions to Executive Editor Scott Arnold at [email protected].