Health-Care-Associated-Infection Control: Reducing Airborne Pathogens
In the United States, health-care-associated infections (HAIs), also known as hospital-acquired infections and nosocomial infections, kill more people than AIDS, breast cancer, and automobile accidents combined. The most dangerous HAI pathogens are those with the potential to spread by air.1 Many of these pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), are called “superbugs” because they are virtually invincible to standard drug treatments. Their airborne transmission through a non-immune population can be rapid and pervasive.2
According to the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO), antibiotic-resistant HAIs are on the rise. This article discusses several methods of controlling their airborne transmission.
Infection Controls
There are four methods of reducing concentrations of airborne infectious agents: dilution, filtration, pressurization, and disinfection. Following is a brief discussion of each.
Dilution. Dilution ventilation helps to control infectious particles by introducing outdoor air—usually, two to five air changes per hour (ACH)—to dilute space air and then exhausting that amount as contaminated air. If 100 percent of all supply air were outdoor air, nearly that amount of airborne infectious particles might be exhausted. However, conditioning that amount of outdoor air would be cost-prohibitive.
Filtration. Filtration should be considered the first line of defense against infectious agents, as it removes a large percentage of them with every complete air change. The higher the filter efficiency and/or air-change rate, the larger the number of infectious agents removed per pass.
Current design guidelines suggest as many as 25 (up to five outdoor air) ACH for new facilities, depending on the spaces served. Because most airborne pathogens originate from people, exceeding 25 ACH brings diminishing returns, which can be seen in this simple equation:
Concentration = generation rate ÷ (flow rate × filter efficiency)
If flow rate or filter efficiency is increased, particle concentrations will decrease mathematically. However, the algorithm favors reduction of in-room source or generation rates of infectious agents, often referred to as source control or source reduction.
Pressurization. Pressurization protects against cross contamination—that is, air in one space contaminating that in another. This is of great importance in health-care settings, but very difficult to control.
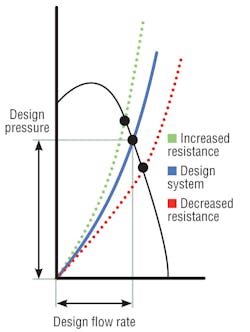
Air-handler design resistance is the sum of all pressure losses through a system, including elbows, dampers, filters, and coils. The shape of a resistance curve (Figure 1) changes when pressure losses change.3 For instance, as filter resistance increases, system air volume decreases. However, coil pressure drop also may increase, even double, resulting in higher system pressure drop. Curves show that as system total resistance increases, air volume and system pressure capability decrease (at a constant fan rotation). This reduction in “design pressure” often results from reduced coil-cleaning procedures, and its effect—reduced air volume and relative room pressure capability—is not at all obvious. When one space is said to be negative in relation to others, the adjoining spaces are assumed to be “positive.” A higher coil pressure drop may negate that and permit the infiltration of contaminated air.
Measuring pressure drop across a coil and comparing it to as-built design data or, better yet, manufacturer performance data is one way to determine a loss in airflow. If coil pressure drop is higher, airflow should be measured to confirm it matches as-built criteria. If a coil is fouled and air pressure compromised, the 2011 edition of ASHRAE Handbook—HVAC Applications recommends the installation of ultraviolet C (UV-C) lamps to continuously clean the coil. This ensures proper airflow and pressure relationships and restores as-built cooling capacity (i.e., heat-transfer characteristics). According to ASHRAE, it also eliminates the growth of mold and bacteria in coil plenums, removing the possibility of microbial-products carryover and transfer to conditioned spaces.
Disinfection. Concentrations of airborne bacteria are proportional to the number of people in a room.4,5,6,7 Airborne microbes from occupation and activity settle on and contaminate surfaces. In sensitive areas, WHO8 recommends a limit of 100 colony-forming units (cfu) per cubic meter for bacteria and 50 cfu per cubic meter for fungi. There are no published colony-forming-unit standards in the United States.
While disinfection in health-care settings is one of its oldest uses, UV-C equipment is underutilized today. Seventy years ago, it began being used to disinfect upper air and ventilation air and to sterilize medical equipment and water. Despite measured success, its use began to wane with increased reliance on antibiotics in the late 1950s.
UV-C destroys all microorganisms—science has yet to find one, including superbugs, resistant to the destructive effects of the 254-nm germicidal wavelength—and its use is extremely simple and inexpensive, but like all controls, it alone is not a complete solution.
UV-C for Infection Control
There are three primary means of applying UV-C systems in the fight against infectious agents: upper-air/room placement, coil irradiation, and air-stream disinfection. Upper-air/room systems are installed in spaces, such as above patient beds and in waiting rooms, corridors, and break areas. Coil-irradiation and air-stream-disinfection systems are installed within air-handling units and duct runs. Upper-air/room and HVAC applications are described below.
Upper air/room. The primary objective of upper-air/room placement of UV-C is to interrupt the transmission of airborne infectious agents in patient rooms, waiting rooms, lobbies, stairwells, laundry chutes, and the like.9 Airborne droplets containing infectious agents can remain in room air for 6 min or more. Upper-air UV-C fixtures can destroy those microbes in a matter of seconds. Operating 24 hr a day, upper-air systems also are especially effective at notably reducing the potential viability of surface microbes that settle out of room air.
Humans are the source of airborne agents that infect people.10 Again, upper-air systems intercept microbes where they are generated, thereby controlling them at the source.11 They have been shown to be effective against viruses and bacteria, including chickenpox, measles, mumps, tuberculosis (TB), and cold viruses. Studies of Mycobacterium tuberculosis show they can be equivalent to 10 to 25 ACH.12 In one study,13 guinea pigs were exposed to exhaust air from a TB ward. Thirty-five percent of the controls developed TB infections. Where upper-air UV-C was used, only 9.5 percent—74 percent fewer—developed infections.
Measles and influenza viruses and tuberculosis bacteria are transmitted by air shared between infected and susceptible persons. Studies indicate there are two transmission patterns: (1) within a room and (2) through corridors and ventilation ductwork. Since the 1930s,14,15,16,17,18 numerous experimental studies have demonstrated the efficacy of upper-air UV-C. Additionally, effectiveness has been shown in reducing measles transmission in a school and influenza transmission in a hospital.19 What’s more, fixtures available today provide more output and coverage at less cost and power, utilizing inexpensive and commonly available lamps.
HVAC systems. In HVAC systems, mold and some bacteria can grow in and around cooling coils, drain pans,20 plenum walls, and filters. Growth of microbial deposits leads to coil fouling, which increases coil pressure drop and reduces airflow and heat-exchange efficiency.21 As performance degrades, so does the quality, amount, and pressurization capability of air supplied to conditioned spaces.1,22
Hospital codes call for high-efficiency filters to be located downstream of cooling coils, where they can become damp and even wet from saturated air. As a result, air filters are considered a growth medium for mold and bacteria and a reservoir for infectious-disease agents. A 360-degree UV-C system installed downstream of cooling coils will destroy all microbes in and on both the coils and filters. In a “common” coil-irradiation system, a 360-degree lamp also will kill infectious agents in the air. For many infectious agents, up to a 35 percent kill ratio is achievable, providing a measurable increase in the combined removal rate of coils and filters.22
UV-C Design Guidance
Historically, engineers and facility operators wanting to apply UV-C lacked guidance for system design, sizing, and specification. ASHRAE looked to rectify that by forming a technical committee (TC 2.9, Ultraviolet Air and Surface Treatment) to author chapters in its 2008, 2011, and 2012 handbooks. HVAC trade publications, meanwhile, have published technical articles providing additional design guidance in recent years. These resources provide the guidance needed to design, install, operate, and maintain successful UV-C applications.
Safety and Handling
Opening doors to air-handler fan sections must be minimized because it allows unfiltered air to enter and be dispersed, potentially to sensitive areas, and/or it disrupts pressure relationships in spaces served by the air handler. Shutting down these systems, meanwhile, can disrupt pressure relationships beyond the spaces served. The opening of air-handler fan-section doors and system shutdown, when necessary, should be coordinated with floor nurses so all room doors can be closed beforehand.
UV-C exposure is an effective means of destroying microbes on filter-media surfaces. Glass-media filters are compatible with UV-C, while synthetic-media filters are not.
Caution should be exercised when using unsupported bag-style filters, as they inherently collapse when being replaced, expelling microbes. The Centers for Disease Control and Prevention recommends that all used filters be bagged upon removal to prevent dispersion of microbes during transport.
Facility staffs require training for proper inspection of UV-C systems. Controls should be installed so UV-C systems turn off when air-handler doors are open. Eye and skin protection are needed in the presence of UV-C light.
UV-C lamps are very similar in construction to fluorescent lamps and, therefore, contain trace amounts of mercury. The use of encapsulated lamps is recommended to prevent air-handler contamination should a lamp break. Like fluorescent lamps, UV-C lamps should be replaced and recycled annually.
Summary
UV-C installations are a simple, effective, and relatively inexpensive means of reducing concentrations of airborne and surface pathogens that cause HAIs.
Within patient rooms, waiting rooms, and other spaces where people congregate, upper-air UV-C units kill airborne microorganisms. UV-C lamps can be installed downstream of cooling coils to keep the coils clean and to supplement efforts to keep the air stream and filter surfaces clean.
ASHRAE and articles appearing in HVAC trade publications provide the information needed to size, select, install, operate, and maintain UV-C systems.
References
- Kowalski, W.J. (2005). Aerobiological engineering handbook: Airborne disease and control technologies. New York: McGraw-Hill.
- Weinstein, R.A. (2004, June 3). Planning for epidemics — The lessons of SARS. The New England Journal of Medicine, 350, 2332-2334.
- Greenheck. (1999). Understanding fan system effects. Greenheck Product Application Guide, Fan Application No. FA/100-99.
- Mangram, A.J., Horan, T.C., Pearson, M.L., Silver, L.C., Jarvis, W.R., & HICPAC. (1999, April). Guideline for prevention of surgical site infection, 1999. American Journal of Infection Control, pp. 97–132.
- Duvlis, Z., & Drescher, J. (1980). Investigations on the concentration of air-borne germs in conventionally air-conditioned operating theaters. Zentralbl Bakteriol, 170, 185–198.
- Moggio, M., Goldner, J.L., McCollum, D.E., & Beissinger, S.F. (1979). Wound infections in patients undergoing total hip arthroplasty. Archives of Surgery, 114, 815–823.
- Kundsin, R. (1976). Operating room as a source of wound contamination and infection. National Research Council, National Academy of Sciences, pp. 167–172.
- WHO. (1988). Indoor air quality: Biological contaminants: Report on a WHO meeting, Rautavaara, 29 August - 2 September 1988. Copenhagen, Denmark: World Health Organization, Regional Office for Europe.
- ASHRAE. (2011). ASHRAE handbook—HVAC applications (ch. 60). Atlanta: ASHRAE.
- Macher, J.M. (Ed.). (1998). Bioaerosols: Assessment and control (ch. 9). Cincinnati: American Conference of Governmental Industrial Hygienists.
- First, M.W., Nardell, E.A., Chaisson, W., & Riley, R. (1999). Guidelines for the application of upper-room ultraviolet germicidal irradiation for preventing transmission of airborne contagion - Part II: Design and operation guidance. ASHRAE Transactions, 105, pt. 1.
- Jensen, P.A., Lambert, L.A., Iademarco, M.F., & Ridzon, R. (2005). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. Morbidity and Mortality Weekly Report, pp. 37-38, 70-75.
- Escombe, A.R., et al. (2009). Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLOS Medicine.
- Wells, W.F. (1955). Airborne contagion. New York: New York Academy of Sciences.
- Riley, R.L., & O’Grady, F. (1961). Airborne infection—Transmission and control. New York: Macmillan.
- Miller, S.L., et al. (2002). Efficacy of ultraviolet irradiation in controlling the spread of tuberculosis. NIOSH Contract 200-97-2602.
- Xu, P., et al. (2003, January). Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-scale studies. Atmospheric Environment, pp. 405–419.
- First, M., Rudnick, S., Banahan, K., Vincent, R., & Brickner, P. (2007, March). Fundamental factors affecting upper-room ultraviolet germicidal irradiation - Part I. Experimental. Journal of Occupational and Environmental Hygiene, pp. 321-331.
- McLean, R. (1961). The effect of ultraviolet radiation upon the transmission of epidemic influenza in long-term hospital patients. The American Review of Respiratory Disease, pp. 36–38.
- Levetin, E., Shaughnessy, R., Rogers, C.A., & Scheir, R. (2001, August). Effectiveness of germicidal UV radiation for reducing fungal contamination within air-handling units. Applied and Environmental Microbiology, pp. 3712-3715.
- Montgomery, R.D., & Baker, R. (2006, November). Study verifies coil cleaning saves energy. ASHRAE Journal, pp. 34-36.
- Kowalski, W. (2009). Ultraviolet germicidal irradiation handbook: UVGI for air and surface disinfection. Berlin: Springer.
The president of UV Resources and past director of Farr Co., Forrest Fencl is an ASHRAE Fellow, a former ASHRAE Distinguished Lecturer, and a member of the International Ultraviolet Association and the Illuminating Engineering Society. He can be contacted at [email protected].
Did you find this article useful? Send comments and suggestions to Executive Editor Scott Arnold at [email protected].