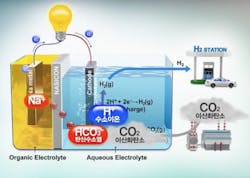
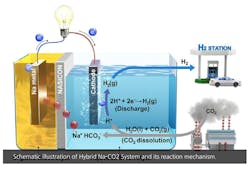
Salt + H2O - CO2 = Electricity + H2
Most of us are probably at least noddingly familiar with fuel cells, since they were widely touted by NASA for their applications in space craft and satellites. Some of us in the HVAC industry may even be using forklifts running on fuel cells. And there are also fuel cell-powered cars and buses out there.
However, many experts – including such luminaries as Elon Musk, who calls them “fool cells” – believe that their applications are limited by the cost of producing hydrogen, which most fuel cells rely on, and the danger of transporting hydrogen due to its flammability (See, 'Hindenburg'). Typically, a fuel cell uses hydrogen and oxygen in an electrochemical reaction to generate electricity, with water as the byproduct. When hydrogen is burned with oxygen, it’s a zero-emission “clean” fuel.
A regular contributor to HPAC Engineering and a member of its editorial advisory board, the author is a principal at Sustainable Performance Solutions LLC, a south Florida-based engineering firm focusing on energy and sustainability.
About the Author
Larry Clark
A member of HPAC Engineering’s Editorial Advisory Board, Lawrence (Larry) Clark, QCxP, GGP, LEED AP+, is principal of Sustainable Performance Solutions LLC, a South Florida-based engineering firm focused on energy and sustainability consulting. He has more than two dozen published articles on HVAC- and energy-related topics to his credit and frequently lectures on green-building best practices, central-energy-plant optimization, and demand-controlled ventilation.