Optimizing Heat Transfer in Steam Heating Systems
Has your facility experienced a steam-system (air heater, fuel-oil heater, water heater, or platen-type press) heating problem that engineering and maintenance has been unable to resolve? This article provides a practical and theoretical discussion about improving steam systems.
Steam Quality and Heat Transfer
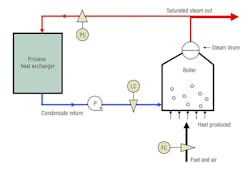
Steam is a gas consisting of water vapor. The vapor contains a significant amount of thermal energy commonly measured in British thermal units. In many facilities, steam is used to heat water, air, fuel oil, and many other types of liquids and gases. It also can be used in platen presses and autoclaves to heat and cure solid materials, plastics, painted parts, etc. For most of these applications, steam is produced in a gas-fired boiler at pressures from 15 to 250 psig (gauge).
The total energy contained in steam at these pressures can range from about 1,160 Btu per pound saturated to more than 1,400 Btu per pound, if superheated. An important property of steam is latent heat (i.e., the energy transfer that instantly occurs as water changes from liquid to gas or from gas to liquid). This change of state is known as evaporation or condensation heat transfer. The word “latent” is French and means “cannot be measured.” Conversely, hot water can produce only 1 Btu per pound, yet is easily measured by temperature change.
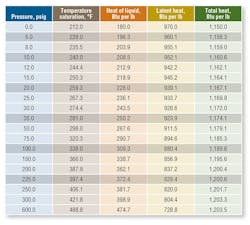
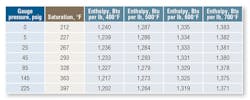
Table 1 shows properties of steam that may be used in process heating systems. Note latent heat constitutes a major portion of the total energy contained in steam. Moreover, there is significantly more latent heat in steam at lower pressures than steam at higher pressures. As a result, designers of condensing heat-transfer equipment often use low-pressure steam (just high enough to make the condensate traps and piping system operate economically and effectively).
We can determine the conditions before and after a “change of state,” but must refer to these thermodynamic tables to determine the energy transfer that occurred.
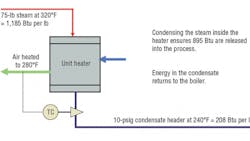
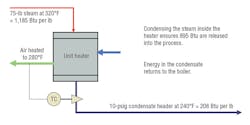
Note that while the total energy in steam increases with pressure, latent heat (condensation heat-transfer energy) is highest at the lowest pressure. Providers of heat exchangers, platen presses, etc. normally design systems to operate at steam pressures of about 10 to 30 psig in the heat exchanger (high latent heat transfer, but at a lower temperature). If a higher temperature is required for baking or curing, then the steam pressure in the exchanger needs to be higher. Accordingly, more steam is used. It is important, then, to recognize from Table 1 that, as steam enters an exchanger at 10 psig:
- It contains about 1,160 Btu per pound of thermal energy.
- The latent heat of condensation (952 Btu) is the secret to successful, efficient operation of the process.
- The condensate produced will contain about 208 Btu of residual energy that needs to be captured and returned to the boiler system for re-use.
Steam entering an exchanger must be condensed from a gas to a liquid (condensate) to release 950 Btu of energy to the system. This is crucial. The process must be controlled (generally, the temperature of the gas or liquid being heated is monitored) to ensure proper pressure or temperature inside the heat exchanger. Some products are a “batch” heated for a specific time at a nominal ambient temperature inside a vessel.
Superheated and Poor-Quality Steam
Steam is a unique gas. It also can be superheated to a temperature above the vaporization state. Superheated steam is produced by passing the steam line back into the boiler heating zone to absorb more energy. However, for the steam to condense and release its latent heat efficiently, it must be very near a saturation-temperature condition entering the heat exchanger. Superheated steam also can be produced by reducing the pressure of a higher-pressure steam supply through a control valve.
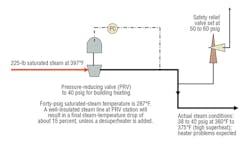
Note in Table 1 the saturation temperature of steam at 225 psig is 397.4°F, which is nearly 160°F above the saturation temperature at 10 psig (240.0°F). Supplying saturated steam from a boiler operating at 225 psig through a pressure-reducing valve will cause a steam superheat condition at the lower pressure (because direct expansion does not release all superheat energy). Thus, it is normal to install a desuperheater to lower steam temperature to near saturation conditions at lower pressure. This is accomplished by spraying water (pure condensate) into a steam line. The condensate evaporates and absorbs thermal energy, which, in turn, reduces steam temperature. Quality desuperheater equipment and accurate controls are necessary to produce ideal steam conditions.
If, because of boiler design or piping-system operational anomalies, steam has a high level of superheat, it could pass through an exchanger before it condenses and releases energy. Partial-condensation heat transfer then would occur. A smaller amount of thermal energy would be absorbed by the system, and heat transfer would be less effective. Exchanger overheating could occur, resulting in poor efficiency, water hammer in the condensate-piping system, and even product-quality concerns.
Generally, steam should enter a heat exchanger at a superheat condition of no more than 10°F. For example, for an 8-psig operating pressure, steam temperature should be no more than 245°F. This is an estimated temperature setting (to produce latent heat transfer) based on system piping configuration. As steam passes through the valves before an exchanger, some expansion cooling occurs. Ideal condensation heat transfer, however, depends on many issues.
A second condition of supply steam involves the presence of a large amount of condensate (i.e., partial condensation occurred prior to the heat exchanger, perhaps because of boiler problems, poor piping insulation, wet steam from another source, defective steam condensate traps, a malfunctioning desuperheater, instrument control problems, etc.).
Steam-heat-exchanger designers, thus, have a unique challenge: develop a system that will cause condensation heat transfer to occur inside a vessel regardless of changing operating conditions. Supply-pressure changes, a stuck control valve, poor insulation, bad traps, etc. can affect system operation, resulting in product anomalies and/or wasted energy.
The goal of designers is to transport superheated steam through a piping system or process without incurring a change of state (i.e., to eliminate condensation heat transfer).
Thermodynamic Properties of Steam
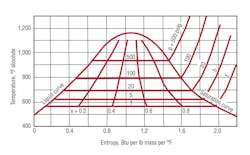
Figure 1 shows the amount of latent heat transfer that occurs as steam condenses at higher pressures and temperatures. Enthalpy change is the energy transferred during condensation. It can be represented visually by the length of the horizontal line from right (saturated-steam line) to left (100-percent-liquid-condensate line). Entropy, a condition of system reversible disorder, can indicate the relative condensation conditions as the process occurs (wet steam). Remember, the higher the steam pressure at which condensation occurs, the lower the amount of energy that is released.
Wet steam produces less condensation heat transfer. It is an indication condensation heat transfer has begun.
Superheated-steam conditions are shown to the right of the saturation line. Superheated steam generally does not provide a high level of heat transfer or efficient heating. It must first be cooled to saturation conditions for condensation to occur.
Reducing superheated-steam conditions (in a pure heat-transfer process) is difficult without performing mechanical work on the surrounding system (i.e., removing some superheat energy).
Practical Steam-Heat-Exchanger Application
It is very important to know the pressure and temperature of the steam entering an exchanger. Robust (utility-grade) instruments and controls often are the answer.
Optimizing heat-transfer performance and energy consumption of a system generally requires inspection and testing. Upon achieving ideal steam conditions inside an exchanger, a “step change” in process performance (i.e., the “sweet spot”) will be obvious.
Conditions that can affect a system and reduce process efficiency are:
- Fluctuating steam supply pressure.
- Superheated steam supply.
- Impure desuperheater water.
- Defective steam traps, control valves, control instruments.
- Inadequate supply of steam.
- Improperly sized heat exchanger.
- Steam leaks at exchanger pipe connections.
- Poor piping and heat-exchanger insulation.
- Cold air or water contacting the exchanger.
Summary
Optimum performance of a steam-heat-exchanger process requires a certain set of design conditions, equipment in good working order, and proper ambient conditions. Both steam pressure and steam temperature need to be measured and controlled. Generally, problematic issues can be identified through brief analysis of a system by a specialist.
Reference
1) Marks, L.S., & Baumeister, T. (1958). Mark’s mechanical engineers’ handbook (6th ed.). New York: McGraw-Hill.
The president of JoGar Energy Services, provider of on-site technical reviews, inspection services, and training seminars, Gary W. Wamsley, PE, CEM, has more than 40 years of plant-operation and management experience in the tire, aerospace, and paper industries. He is a member of ASME, the American Institute of Plant Engineers, the National Society of Professional Engineers, and the Association of Energy Engineers, as well as HPAC Engineering’s Editorial Advisory Board.
Did you find this article useful? Send comments and suggestions to Executive Editor Scott Arnold at [email protected].