Design of a Specialized Airborne-Infection-Isolation Suite
A biocontainment patient-care unit (BPCU) is a facility designed and operated to maximize patient care with appropriate infection-control practices and procedures. A BPCU is secure, is physically separated from other patient-care areas, and has special air-handling systems.1
The National Institutes of Health's new Special Clinical Studies Unit (SCSU) in Bethesda, Md., is a highly specialized BPCU, housing patients with extremely infectious diseases transmissible by respiratory aerosolization, direct contact with primary body fluids, or dried infectious particles from body fluids. The SCSU has three patient rooms plus an occupational-exposure isolation room (OEIR). It is equipped to meet all clinical-care needs, including basic medical observation, minor surgical procedures, and intensive care. Thus, planning had to address housekeeping, security, emergency evacuation, and the use of experimental therapeutics.
This article will discuss design criteria used for the SCSU, which can be applied for any other type of airborne-infection-isolation suite (AIIS).
General Design Features
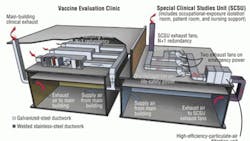
The SCSU receives air via the main clinical supply-air system, which is served by air-handling units located in another part of the building. This is a manifolded system providing supply air to all patient rooms and support spaces (Figure 1).
All clinical spaces are served by a non-recirculating (100-percent exhaust) HVAC system. All general clinical spaces, as well as the adjacent Vaccine Evaluation Clinic (VEC), are served by general exhaust systems consisting of multiple exhaust fans connected to a common exhaust-air manifold. Dedicated isolation-room exhaust systems consist of multiple exhaust fans connected to a common exhaust-air manifold. If one fan fails, the remaining fans compensate to provide the required design exhaust-air quantity.
The VEC is exhausted through the general clinical exhaust system, while the SCSU is exhausted through a dedicated system. Exhaust air from all SCSU spaces, including the OEIR, is manifolded in the dedicated exhaust system.
The dedicated system is equipped with high-efficiency-particulate-air (HEPA) filters prior to exhaust fans and discharges outside (Figure 2).
At both entrances to the SCSU are vestibules maintained at negative pressure relative to the adjoining corridors. The OEIR has an anteroom maintained at negative pressure relative to the rest of the SCSU to mitigate the risk of release of an airborne agent. The negative pressure of the patient rooms, the OEIR, and the SCSU is monitored and alarmed at the nurses' station.
SCSU Design Features
Design features specific to the SCSU include:
- Walls (outside of the OEIR) sealed to minimize uncontrolled air movement to and from adjacent spaces, sealed piping penetrations, and openings in electrical boxes and other component device boxes sealed with caulking.
- Self-closing exit doors.
- Balanced ventilation air to maintain negative pressure (inflow of air) relative to the corridor (between public corridor and the SCSU).
- Exhaust-air grilles located low on patient-room walls.
- Pressure-monitoring system continuously indicating airflow from adjacent spaces into the SCSU.
- Displays of relative space pressurization at the corridor and vestibules.
- Remote alarms at the nurses’ station.
- Twelve-air-changes-per-hour (ACH) minimum ventilation rate of non-recirculating air (100-percent outdoor air) for the SCSU and OEIR.
- Dedicated toilet, bath, and hand-washing facilities in each patient room.
- Dedicated exhaust-air system serving the SCSU and OEIR.
The dedicated constant-volume exhaust system consists of three variable-speed exhaust fans. Each fan is designed at 50 percent of capacity to provide the required level of redundancy and reliability and 20-percent reserve-airflow capacity to account for final air-balance adjustments and future needs. The fans operate continuously, their speed controlled with variable-frequency drives based on a static-pressure sensor in the exhaust ductwork. Discharge from the fans is manifolded to ensure a high stack-discharge velocity.
One of the exhaust fans receives life-safety branch power; the other two receive general standby-equipment branch power. In the event of normal power loss, life-safety power is restored within 10 sec, and the first fan is re-engaged and ramps up to satisfy required minimum exhaust and directional airflows. Shortly thereafter, power to the other fans is restored, and the fans are re-engaged. Air distribution to individual spaces/rooms is by constant- and/or variable-volume, pressure-independent supply and exhaust air terminals to each temperature- and/or pressure-control zone. Pairs of supply and exhaust venturi-style air-terminal units track airflow to ensure the specified pressurization (directional airflow) is maintained. The only prerequisite for the air terminals to operate properly is sufficient duct static pressure. This is monitored via differential-pressure switches across each air valve. Additionally, automatic bubble-tight isolation dampers are provided in the supply duct to each zone.
All exhaust air-terminal units are low-leakage (less than 3 cfm at 1 in. water), with high-speed actuators (less-than-1-sec full response time) to stay within 5 percent of airflow set point. The air-terminal units' controllers are linked between supply and exhaust. If duct static pressure falls below levels required for accurate tracking, the supply-air bubble-tight dampers close, and the exhaust-air valves are commanded to a minimum position, maintaining directional airflow.
The control devices operate autonomously, even in the event communications with the overall building control system are disrupted. All of the controllers and bubble-tight dampers are provided uninterruptible power to ensure the air-terminal units track each other to maintain the specified airflow differential. All serviceable components, such as air terminals, exhaust valves, and reheat coils, are located in the interstitial space outside of the patient-care/containment area.
All exhaust ductwork for the SCSU and negative-pressure examination room on the room side of the HEPA filter is made of welded stainless steel (Figure 3). The supply ductwork is made of galvanized steel augmented with welded stainless steel. Bubble-tight dampers are provided on all supply ducts branching from the interstitial level to SCSU patient rooms and the OEIR. These are fast-acting pneumatic actuators that close upon a loss of exhaust airflow. Ductwork from the bubble-tight dampers to air devices is made of welded stainless steel.
Backflow preventers are installed in domestic-water piping running to the SCSU. When the SCSU is in isolation mode, oxygen, medical vacuum, and medical air are provided via local dedicated units. HEPA filtration is provided on the main plumbing vents leaving the SCSU.
Emergency power is provided for critical branch, life-safety, and standby equipment in the SCSU and for exhaust fans on the interstitial level as appropriate.
Electrical services for the OEIR are sealed airtight to minimize outside penetrations. A third inner layer of sheetrock prevents air migration that can occur as the result of required utility boxes and associated penetrations.
Room Control Sequences
Under normal conditions, supply- and exhaust-air valves operate at "constant" airflow set points. The fixed differential between supply- and exhaust-airflow set points provides directional airflow and room pressurization. For example, an exhaust-airflow set point of 700 cfm and supply-airflow set point of 600 cfm provides 100-cfm inward airflow, maintaining a room at negative pressure. As long as the "measured" differential pressure across the supply- and exhaust-air valves is within an acceptable range (0.6 in. wg to 3.0 in. wg), air valves maintain their respective constant-airflow set points, maintaining directional airflow and room pressurization. Differential-pressure switches across each supply- and exhaust-air valve continuously monitor airflow.
If the differential pressure across either the supply- or exhaust-air valve in any room drops below 0.3 in. wg, the bubble-tight damper on the supply duct to that room closes immediately. The supply-airflow and exhaust-airflow set points are reset to emergency-mode minimum values. When the main supply-duct static pressure and main exhaust-duct static pressure rise above 0.85 in. wg, the bubble-tight damper opens, supply- and exhaust-airflow set points are reset to their normal values, and the room reverts to normal operation. The room control sequences are illustrated in figures 4-6.
Particle Dynamics
Clinically applicable distinctions are made between short-range airborne-infection routes (between individuals less than 3.28 ft apart) and long-range routes (within a room, between rooms, or between individuals greater than 3.28 ft apart). Small droplets may participate in short-range transmission, but are more likely than large droplets to evaporate to become droplet nuclei. True long-range aerosol transmission becomes possible when droplets of infectious material are small enough to remain airborne almost indefinitely and be transmitted over long distances. There is essential agreement that particles with an aerodynamic diameter of 5 ìm or less are aerosols, while particles with an aerodynamic diameter of 20 ìm are large droplets.
Methodology
For the SCSU, computational fluid dynamics and particle tracking were used to study the influence of ventilation flow rate (12 ACH and 16 ACH), exhaust location (high exhaust, low exhaust, and the combination of high and low exhausts), and patient position (sitting and lying) on the removal of aerosol generated by a cough.
The model suite consisted of three rooms—main isolation room with patient bed, bathroom, and anteroom—connected via door gaps.
A patient's cough was assumed to release 3,000 droplet nuclei with diameters of 3 µm.2,3 Further, the particles were assumed to be 93°F when released from the mouth.4
Results
Three thousand particles were tracked individually for 5 min, with the number of particles removed from the room recorded every minute. The location of each particle remaining in the room was recorded. Particular attention was paid to the number of particles remaining in the breathing zone of the main room and the breathing zone around the bed where health-care workers were most likely to be. The breathing zone of the main room was defined as the space 3.6 ft to 5.75 ft from the floor; the breathing zone around the bed was defined as the space 9 in. from the edges of, and 3.6 ft to 5.75 ft above, the bed (Figure 7).
Table 1 shows the percentage of particles removed from the suite 3 min and 5 min after a cough and the percentages of particles remaining in the bathroom, the main room, and the breathing zone of the main room. During the first 3 min, the system removed 13 percent to 45 percent of the 3,000 particles. Within 5 min, the percentage of particles removed increased to 25 to 55 percent, which is consistent with estimates5 that, assuming a well-mixing condition, 99 percent of particles will be removed after 9 min at 16 ACH. Note the percentage of particles in the bathroom is much higher when a sitting patient coughs than when a lying patient coughs. The reason is the distribution of particles in the air current and the location of the bathroom.
Figure 8 shows the number of particles removed 3 min and 5 min after the cough of a sitting patient and a lying patient. The combination of low and high exhausts was least effective in removing particles. Generally, with the exception of Case 10, the most particles were removed with low exhaust and high flow. With a lying patient, 12 ACH (Case 10) removed more particles than 16 ACH (Case 12) with high exhaust. This can be explained by the flow pattern above the patient, determined by the downward forced convection from the ceiling diffusers above the bed and the upward flow of the cough from the mouth of the lying patient.
With greater downward flow (16 ACH, rather than 12 ACH) from the ceiling diffuser, the upward movement of droplets carried by the cough of a lying patient could be suppressed and, thus, have difficulty reaching high exhausts.
For a sitting patient, particles in the breathing zone generally were reduced from minutes 3 to 5 (Figure 9). For a lying patient, they sometimes increased, depending on the ventilation system and flow rate. Figure 10 shows the combination of low and high exhausts resulted in a higher number of particles in the breathing zone around the bed, especially in the cases (6 and 8) of a lying patient. High exhausts with a high flow rate (Case 12) did not remove particles around the bed effectively. Figure 10 indicates fewer particles around the bed with low exhausts.
The number of particles below the breathing zone was higher with low exhausts than with high exhausts (Figure 11). High exhausts with high flow rates (Case 12) did not seem to remove particles around the bed effectively. The number of particles above the breathing zone was higher with a lying patient regardless of flow rate and exhaust location (Figure 12). This was attributed primarily to the initial upward momentum of the cough jet.
Conclusions
Conclusions that can be drawn from the study include:
- Low exhaust outperforms other exhaust locations in terms of particle removal and number of particles remaining around a bed.
- Increasing ventilation flow from 12 to 16 ACH generally helps to remove particles from an isolation room (except in cases of high exhaust and a lying patient coughing), but not necessarily a breathing zone.
- The number of particles in a bathroom resulting from a cough in a main room is dependent on air-current particle distribution and bathroom location.
Standard operating procedures are extremely important and should be developed as part of the planning process, with consideration given to facility purpose and features.
References
1) Smith, P.W., et al. (2006). Designing a biocontainment unit to care for patients with serious communicable diseases: A consensus statement. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science, 4, 351-365. Retrieved from http://www.eunid.eu/public/bioconteniment%20unit.pdf
2) Fennelly, K.P., Martyny, J.W., Fulton, K.E., Orme, I.M., Cave, D.M., & Heifets, L.B. (2004). Cough-generated aerosols of Mycobacterium tuberculosis: A new method to study infectiousness. American Journal of Respiratory and Critical Care Medicine, 169, 604-609.
3) Fitzgerald, D., & Haas, D.W. Mycobacterium tuberculosis. In: Mandell, Douglas, and Bennett's principles and practice of infectious diseases (6th ed.). (2005). Philadelphia: Churchill Livingstone.
4) Hoppe, P. (1981). Temperature of expired air under varying climatic conditions. International Journal of Biometeorology, 25, 127-132.
5) Centers for Disease Control and Prevention. (2005, December 30). Guidelines for preventing the transmission of mycobacterium tuberculosis in health-care settings, 2005. Morbidity and Mortality Weekly Report, 54, 20.
Director of the National Institutes of Health's (NIH's) Division of Technical Resources, Farhad Memarzadeh, PhD, PE, consults on matters related to biocontainment and medical research laboratories around the world. He has written four books and more than 60 scientific research and technical papers published in peer-reviewed journals and been a guest and keynote speaker for more than 50 international scientific and engineering conferences and symposia. Deborah E. Wilson, DrPH, CBSP, is director of the NIH's Division of Occupational Health and Safety and founder and director of the National Biosafety and Biocontainment Training Program. She is a career U.S. Public Health Service Commissioned Officer. Krishnan Ramesh, PE, is a managing principal of Affiliated Engineers Inc. He has extensive experience planning, engineering, and designing biological and chemical research laboratories and vivaria nationwide and is a technical contributor to the NIH Design Requirements Manual for Biomedical Laboratories and Animal Research Facilities.
Did you find this article useful? Send comments and suggestions to Executive Editor Scott Arnold at [email protected].